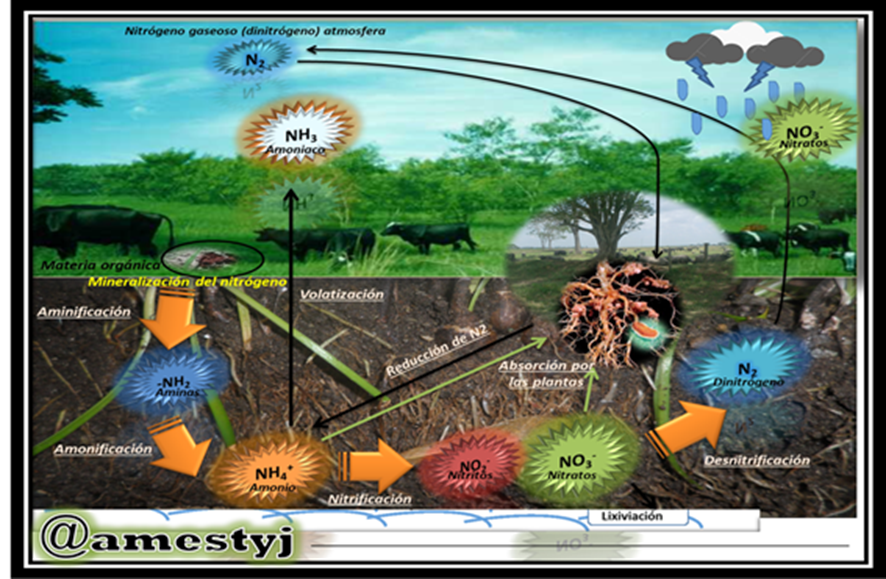
The same author points out that most of the nitrogen present in the soil is found in organic forms, constituting between 90% and 98% of the total nitrogen, these forms are the result of the decomposition of organic matter, including plant and animal waste, microbial biomass and humic substances, organic nitrogen is not directly available to plants and must be mineralized by soil microorganisms to become assimilable inorganic forms. The main forms of organic nitrogen in soil include:
- Proteins and Amino Acids: They are the most abundant nitrogenous components in living organisms and their residues. the breakdown of proteins releases amino acids, which can then be transformed by microorganisms.
- Humic Substances: They are complex organic polymers and resistant to decomposition, formed during the humification of organic matter. They contain a significant amount of organic nitrogen that is slowly released through mineralization.
- Microbial Biomass: Soil microorganisms (bacteria, fungi, actinomycetes) contain a considerable amount of nitrogen in their cells, the death and decomposition of these microorganisms release organic nitrogen that can be mineralized.
- Ammonium (nh₄⁺): It is the first inorganic form of nitrogen produced during the mineralization of organic matter (ammonification). Ammonium has a positive charge and tends to adsorb to clay particles and soil organic matter, which reduces its mobility and protects it from leaching, plants can absorb ammonium directly, although in many soils it is quickly converted to nitrate.
- Nitrate (no₃⁻): It is the predominant form of inorganic nitrogen in most well-aerated soils. It is produced through nitrification, a two-stage microbial process in which ammonium is oxidized first to nitrite (NO₂⁻) by bacteria of the genus Nitrosomonas and then to nitrate by bacteria of the genus Nitrobacter. Nitrate has a negative charge and is highly mobile in the soil, which makes it more susceptible to leaching (loss by washing with rainwater or irrigation). However, it is also the preferred form of nitrogen by many plants due to its high solubility and easy absorption.
- Nitrite (NO₂⁻): It is an intermediate in the nitrification process and is usually found in very low concentrations in the soil due to its rapid conversion to nitrate. Nitrite is toxic to plants and microorganisms in high concentrations.
iv class="text-justify">On the other hand, we have biological nitrogen fixation, which is the conversion of atmospheric nitrogen (N₂) into nitrogenous organic forms by certain microorganisms (bacteria and cyanobacteria), either free-living or in symbiosis with plants (such as legumes and bacteria of the genus Rhizobium). atmospheric nitrogen fixation, electric discharges (lightning strikes) can convert small amounts of atmospheric nitrogen into nitrogen oxides, which then reach the soil with rain and become nitrate., absorption by plants the roots of plants mainly absorb nitrate and ammonium from the soil through active transport processes.