Have you ever dreamed of renewing your body? According to a newspaper magazine, one of five Germans would like to improve their bodies. In fact, when you are from the US or Brasilia the probability is even higher. One-third of all cosmetic surgeries are made in these two countries.
Besides lifting, sucking, pumping or whatever, the time is almost right to renew even the last corner of your body. The development of artificial organs, with huge potential for modern society, is almost completed. Today guts, mini brains, and even a beating heart can be generated in Petri dishes. Further, even legs and arms are under discussion. Let’s see how far the scientists are.
Welcome to my (may be delayed) anniversary post.
It’s more than a year ago since I came here. The Bitcoin drives crazy, Steem is a bit lazy, but I still have a lot of fun here. I was thinking about a long time what could be the best topic for an anniversary post and finally, I decided to research something about artificial organs.
At first, I have to be honest, new extremities are probably still science-fiction…
… even though some creatures already disentangled the mystery for renewing their extremities. Let’s talk about the Axolotl.
 Fig. 1: An Axolotl with five instead of four legs. Thanks to Dr. Isabelle Kolbe for her permission to use this. No Copyright restrictions! Free use allowed!
Fig. 1: An Axolotl with five instead of four legs. Thanks to Dr. Isabelle Kolbe for her permission to use this. No Copyright restrictions! Free use allowed!
Axolotls (an amphibian) can easily create new legs or arms. In this picture, you can see a little stubby leg (blue arrow). This leg has grown after the Axolotl was accidentally attacked by some of his friends. As a consequence, he renewed his normal leg and obtained one leg in addition.
The mechanism behind regenerating extremities in Axolotls was figured out a previously by scientists from Dresden (Germany) [1]. For a long time, it was assumed that cells in the arms know that they are “arm cells”. That means “arms cells“, can only create arms and nothing else. But the opposite is the case. Every cell of an Axolotl can create every body part. The only prerequisite is that they are in the right part of the body. After a while, they recognize where they are and start to differentiate in the area of choice. The scientists were able to show that after transplantation of cells from a region in another region the cells reprogrammed themselves and then becoming part of the new destiny. The trigger for this is still unknown, probably signals from immune cells controlling the fade of the transplanted cells. Anyway, the scientist found the genetic program which is responsible for this interesting “freak of nature”. Maybe some of the more nerdish readers already assumed it: Of course the hox genes are responsible [1]. Hox genes are evolutionary very old and highly conserved (Fig.2)[2]. Flies already have hox genes (called hom-c) and even jellyfishes, plants or fungi carrying related genes [2, 3].
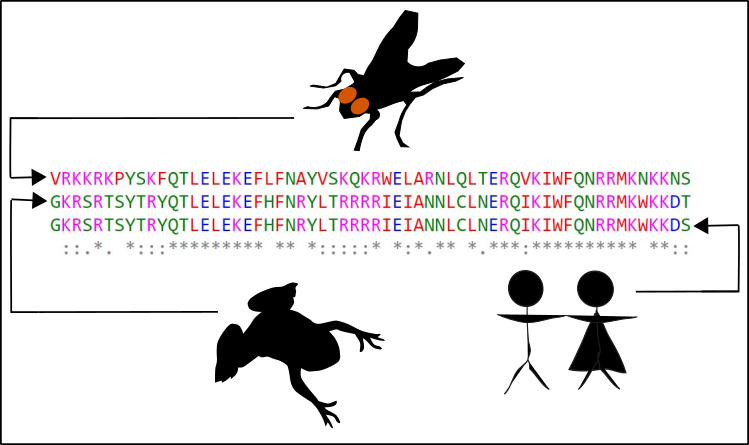 Fig. 2: hox genes are highly conserved. The hox genes, are being transcripted for enabling the cell to translate the hox gene information into a corresponding protein. Within the protein you can find various domains, responsible for DNA binding. This binding is the prerequisite for controlling the activity of other genes. In the figure, you can see the alignment of amino acid sequences (each letter is a different amino acid) of one the DNA binding domains (more specifically the homeodomain). You can see no big difference between flies (Drosophila melanogaster), clawed frogs (Xenopus leavis) and humans (Homo sapiens). If you want to try this by yourself, maybe with a protein of your choice, this is how it works: You go to NCBI enter the protein and the species of choice (choose "protein" at the left of the input field). Then your browser for "good" sequences. The FASTA of appropriate protein sequences are then copy-pasted somewhere (for instance into a normal text editor file). If you collected enough sequences (three or more) in one file, you go to the EMBL and use the tool CLUSTL Omega. Just paste everything insight the next input field and submit. Done! Now you can have fun! Made by Chapper - unrestricted use allowed
Fig. 2: hox genes are highly conserved. The hox genes, are being transcripted for enabling the cell to translate the hox gene information into a corresponding protein. Within the protein you can find various domains, responsible for DNA binding. This binding is the prerequisite for controlling the activity of other genes. In the figure, you can see the alignment of amino acid sequences (each letter is a different amino acid) of one the DNA binding domains (more specifically the homeodomain). You can see no big difference between flies (Drosophila melanogaster), clawed frogs (Xenopus leavis) and humans (Homo sapiens). If you want to try this by yourself, maybe with a protein of your choice, this is how it works: You go to NCBI enter the protein and the species of choice (choose "protein" at the left of the input field). Then your browser for "good" sequences. The FASTA of appropriate protein sequences are then copy-pasted somewhere (for instance into a normal text editor file). If you collected enough sequences (three or more) in one file, you go to the EMBL and use the tool CLUSTL Omega. Just paste everything insight the next input field and submit. Done! Now you can have fun! Made by Chapper - unrestricted use allowed
The main task of hox genes is the control of development programs. Therefore, renewing extremities in Axolotls has something to do with the development [2].
To realize something like this in humans could be a bit tricky. You have to activate the right hox gene at the right place. Further, you have to coordinate the entire development cascade with all the factors playing together. Moreover, you have various hox genes but not all of them are activated at the same time. In Axolotls, for instance, you have HOXA9, HOXA11 andHOXA13 which are involved [1]. Maybe mankind will figure out how to control these programs one day, but it will probably take a bit more time than other strategies I describe below.
In contrast, other approaches are not that "complex" like the regeneration of extremities.
One of the main applications, besides looking better (cosmetic surgery), is clearly the curation of serious diseases. The term organ donation is at the moment a controversially discussed issue in Germany. Anyway, in my opinion, this problem could be solved in a few years because there is a lot of progress in the science of regenerative medicine.
Already at the beginning of the 20-century scientists started to breed organs [4]. At first, they took fragments of organs or tissues and cultured them in nutrient solutions. Thereby, they realized that these fragments started to form organ-like structures. These structures are generally called organoids or 3D cell culture. Later on, cell culture techniques have gotten better allowing the scientists to culture various organoids. Eventually, these approaches led to the discovery of various fundamental cell biological aspects concerning the formation of organs and tissues [4].
Some of these discoveries found that „free“ signaling molecules such as TGF-β as well as cell-bound actors such as Laminin are essential for organ formation [4]. These factors are very important for telling the cells what they should do and they should behave.
Laminin is a so-called glycoprotein (protein + sugar) which resides in the extracellular matrix (ECM) [5]. The ECM is a network of proteins and carbohydrates around the cells. This network has manifold functions, among others signal transduction. To realize this, Laminin interacts with the protein Integrin located on the surface of the target cells. After binding, the cell is embedded into the large community with other cells. These events finally lead to the formation of organs or tissues (GIF1)[4].
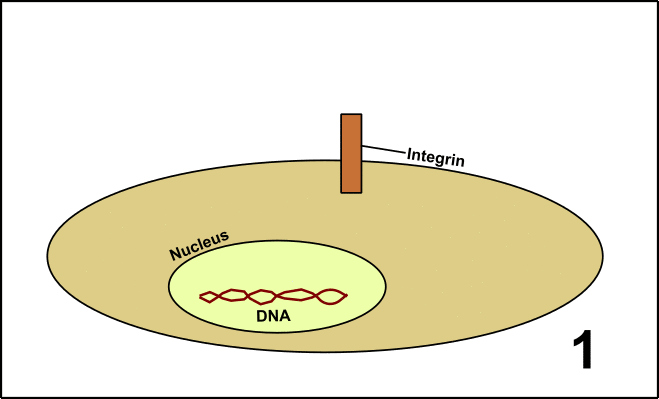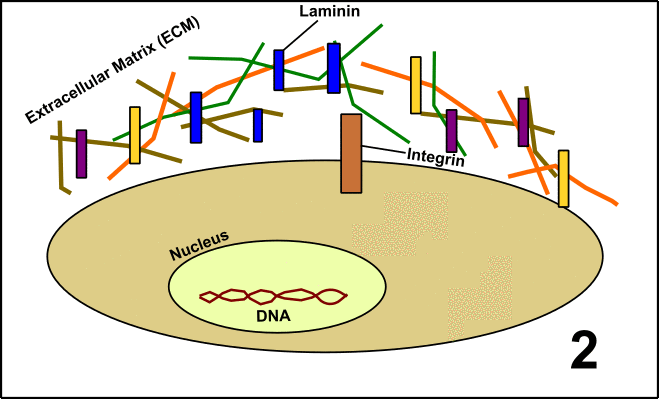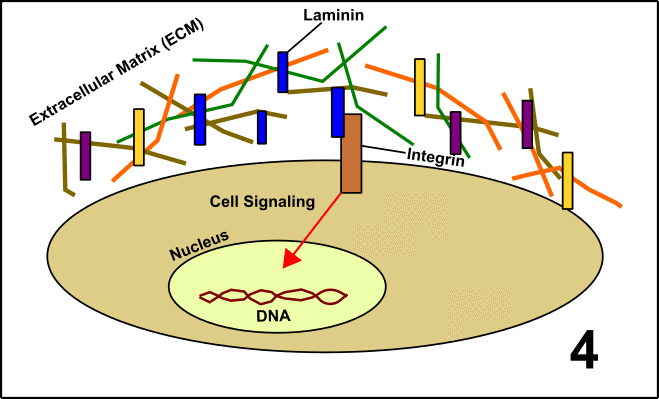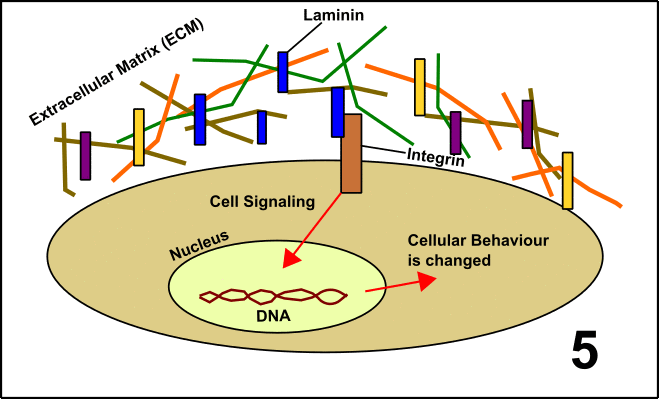 GIF1: Laminin in the extracellular matrix (ECM) determines the fate of cells in a community (organs/tissues). 1. In cells, you can find a nucleus with DNA and the protein Integrin on the cell surface. 2. Around the cells, there is the ECM including Laminin. 3. Laminin interacts with Integrin. 4. Signal transduction is induced. 5. The activity of various genes within the nucleus is altered as a consequence of the signal transduction cascade. The behavior of the cell is changed. Made by Chapper - unrestricted use allowed
GIF1: Laminin in the extracellular matrix (ECM) determines the fate of cells in a community (organs/tissues). 1. In cells, you can find a nucleus with DNA and the protein Integrin on the cell surface. 2. Around the cells, there is the ECM including Laminin. 3. Laminin interacts with Integrin. 4. Signal transduction is induced. 5. The activity of various genes within the nucleus is altered as a consequence of the signal transduction cascade. The behavior of the cell is changed. Made by Chapper - unrestricted use allowed
Very interesting is the story of how the scientists have figured that out. Back in the 1960s or 70s a lot of work was made to find out what the best cell culture conditions are. Therefore, culture vessels were coated with Collagen (another component of the ECM) [5]. It was already accepted that the cells grow better in the presence of Collagen because they are more stable attached to the vessel surface [4]. Nevertheless, even though you have the Collagen coating sometimes cells detaching from the vessel surface. The detached cells, however, didn’t die. In contrast, together with the residual Collagen from the surface, the detached cells grew even better, forming organ-like structures. Today, these organ-like structures are known as Organoids [4]. In comparison to their two-dimensional friends, the organoid cells displayed features no one has observed before. For instance, the mechanism of milk creation in glandular cells was first figured out with organoids [6, 7]. On the basis of such observations, the research of the ECM was intensified. This finally led to the discovery of Laminin. By using antibodies against Laminin and Integrin is was shown, that both molecules are acting together and are prerequisite for organ formation [4]. 3D cell cultures, therefore, is not only a good approach for the study of organ, tissue and, of course, tumor formation. With 3D cell culture, you can also explore the real nature of cells. Keep in mind that cells are not organized in two dimensions, they are part of three-dimensional objects (organs, etc.).
From this point of view, since decades the time of two-dimensional cell culture is already over. 3D cell cultures and organoids will probably triumph in the future.
Nonetheless, full-functioning artificial organs are still hard to achieve.
One big challenge was the establishment of a working 3D cell culture.
Since it is known the ECM is a prerequisite for a working cell culture, 3D cell cultures are cultivated in some kind of a gel. This gel (most prominent since 1977 is the Matrigel [8]) contains numerous factors of the ECM, most important Laminin. With gels, the cells are able to polarize and organize themselves. In the meanwhile, this approach is the standard approach for 3D cell culture.
Another challenge is the origin and the special features of cells.
When I started to get informed about the subject of artificial organs I had the impression that stem cells are absolutely necessary for approaches like this. The main source for stem cells is embryos. However, also in adults, you can also find stem cells. The „adult“ stem cells are responsible for tissue and organ regeneration (e.g. in the muscles [9]). Unfortunately, it is not that easy to get stem cells. Tumors, which have to stem cell-like character, have only little potential for researching organ formation or organ transplantation.
Not that long ago the secret of stem cells was disentangled. Japanese scientist found that every cell could be transformed into a stem cell [10]. It is just important that at least four transcription factors are active.
Four transcription factors are active in stem cells:
- c-Myc
- Oct-3/4
- Sox-2
- KLF4
Because of this Nobel price awarded knowledge, you can take almost every cell in the human body and reprogram them into a so-called induced pluripotent stem cell (iPS cell).
Pluripotent means that the cells are able to differentiate in one of the three embryonal germ layers [11]. The three germ layers are developed after the oocyte became a zygote. The zygote divides several times, forming the Blastula. In a process which is called Gastrulation [12-14] areas on the surface differentiate into the so-called Ectoderm. The Ectoderm is the ancestor of later body surface as well as the nervous system. Inside of the Gastrula, you can find the so-called Entoderm, which later creates the gastrointestinal system. The Mesoderm is de facto between Entoderm and Ectoderm, and can be considered as the origin of the immune system but also creates heart, connective tissues, and muscles (GIF2) [11].
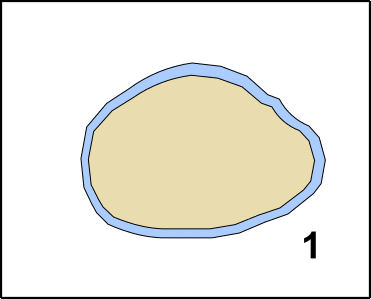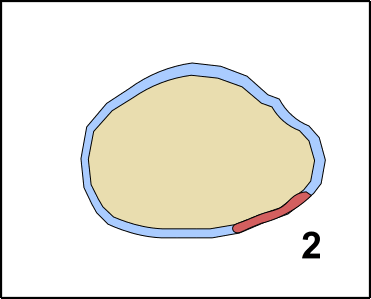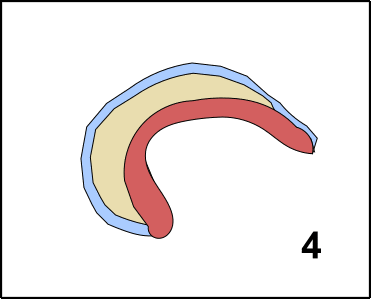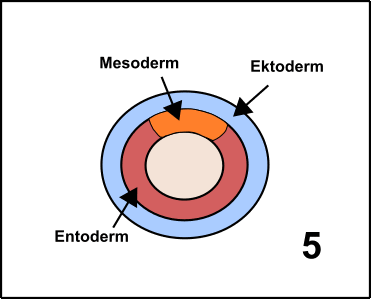 GIF2: Gastrulation. Beginning with the zygote (1) Ectoderm and Entoderm differentiate (2). Step by step the endodermal cells migrate into the oocyte (3 & 4). In the end, a pipe-like structure is formed harboring also the Mesoderm (5). The membrane oft he oocyte belongs to the Ectoderm. Once the „pipe“ is formed, further developmental steps occur. Made by Chapper - unrestricted use allowed
GIF2: Gastrulation. Beginning with the zygote (1) Ectoderm and Entoderm differentiate (2). Step by step the endodermal cells migrate into the oocyte (3 & 4). In the end, a pipe-like structure is formed harboring also the Mesoderm (5). The membrane oft he oocyte belongs to the Ectoderm. Once the „pipe“ is formed, further developmental steps occur. Made by Chapper - unrestricted use allowed
Which kind of germ leave differentiates depends on various transcription factors and signaling molecules [13]. In contrast to the omnipotent stem cells, pluripotent stem cells are no longer able to establish a complete human being. Pluripotent stem cells are solely feasible to create one of the three germ layers or related cell types. Further, you have to be aware because in pluripotent stem cells chromosome ends (Telomeres) are shortenened (more about this here, here and here).
Nonetheless, this technology is the basis for breeding various organoids. Moreover, there are approaches to breed various organoids and interconnect them. This is also called „Body on a Chip“ [4]. Here you have various organoids interconnected via an artificial blood vessel system.
Of course, a big challenge is to create and to maintain a good functioning artificial blood vessel system [15].
Promising approaches to establish blood vessel systems are the use of microchips with tiny channels or nano gels, comparable to Matrigel. In both cases, endothelial cells are used, which eventually form blood vessels. But even larger vessel can be produced and was already successfully transplanted into animals [15].
The main problem, however, is not the breeding of blood vessels or the connection to organoids. More difficult is to transplant blood vessels with artificial organs into living beings. In this case, the organ must be 1) tolerated by the immune system and 2) the body must get connected to the artificial organ as fast as possible [15].
Until today it is not completely understood which signals are necessary to trigger the connection. In the case this doesn’t happen fast enough the artificial organ will become necrotic. With a certain volume of an organ, the presence of a working blood vessel system is unavoidable [15].
One of the most promising approaches is the breeding and transplantation liver fragments [16].
The liver is one of those organs which regenerates pretty well. Nevertheless, the liver often receives damage by poison, alcohol or certain diseases [17, 18]. In these cases, organoids are pretty nice because you can breed and transplant fresh liver fragments fast and easy from your own material. This also lowers the risk of rejection. Further, you don’t have to create an entire liver, even small fragments are sufficient to renew damaged liver parts. Additionally, you don’t need to search or reprogram stem cells. Ordinary liver cells are all you need.
Other approaches plan to transfer induced pluripotent stem cells into pigs [19].
For this purpose, you need transgenic pigs which are no longer able to produce your organ of choice. You can create a transgenic oocyte easily with CRISPR/Cas and then combine them with induced pluripotent stem cells of patients. Subsequently, animals grow which are producing human organs. After a while, you can remove the organs from the animals and transplant them into humans [19].
Of course, the main obstacle is the immunological control while transferring organs from animals into humans. Another severe concern is ethical and juristic issues [20]. Is the pig you kill a 100% pig? Keep in mind that the pig grows up with human organs. Does this fact turn the pig into a human-like creature? Is this a criminal act? And what’s with the recipient? What is the consequence for the recipient? And is it allowed to create hybrid beings such as human pigs?
In my opinion, this approach is probably most promising, but moral, ethics and right should always be the first questions to address.
Organoids will at the end prevail!
Even I have already created organ-like structures without knowing it.
I recognized this while I’m making this post here. Years ago I researched a protein called Clusterin. Clusterin is a glycoprotein acting outside of the cell (such a Laminin) and belongs to the family of molecular chaperones (proteins that take care of other proteins). Therefore, you also find clusterin outside of a cell, also in the ECM. The name Clusterin is derived from the fact that it is feasible to cluster blood cells [21, 22]. At one days, years ago, I was curious whether the cell aggregation really works, thus I did the following thing:
I took some human embryonal kidney cells were we suppressed the production of Clusterin almost completely. At this time CRISPR/Cas wasn’t established, thus we created a stable Knockdown with shRNAs.
Subsequently, I coated a cell culture vessel with a mixture of agarose and agar to prevent the attachment oft he cells. After a few days, I saw that only cells, which were still able to produce Clusterin still displayed aggregate formation. Cells without Clusterin displayed no aggregates anymore (Fig.3).
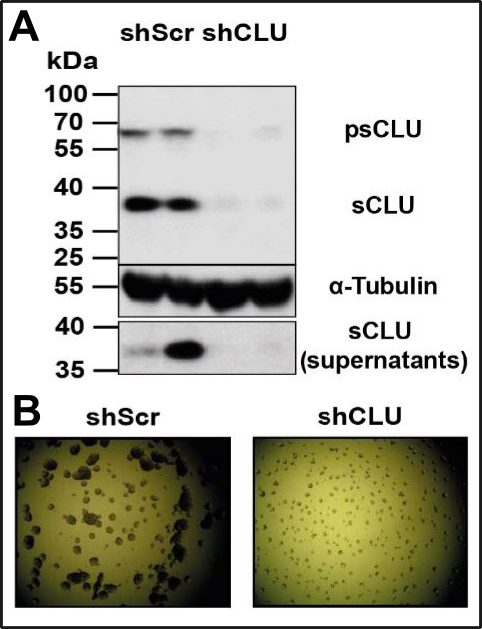 Fig. 3: Chappers organoid breeding! A. Western Blot, where you can see, that our stable Knockdown (shCLU) was successful. sCLU (secretory Clusterin) is the fraction of Clusterin which is present outside of the cell. The explanation of why one of the bands are more intensive (supernatants) I skip (I did a special stimulation there). B. shScr (Scrambled) are control cells which are still able for Clusterin production. You see that cells without Clusterin (shCLU) are evenly distributed as single cells, whereas cells with Clusterin (shScr) are aggregated. Are these organoids? I don‘t know! As described in the text, the culture vessels were coated with an agarose/agar-mix to prevent the attachment of the cells. Made by Chapper - unrestricted use allowed
Fig. 3: Chappers organoid breeding! A. Western Blot, where you can see, that our stable Knockdown (shCLU) was successful. sCLU (secretory Clusterin) is the fraction of Clusterin which is present outside of the cell. The explanation of why one of the bands are more intensive (supernatants) I skip (I did a special stimulation there). B. shScr (Scrambled) are control cells which are still able for Clusterin production. You see that cells without Clusterin (shCLU) are evenly distributed as single cells, whereas cells with Clusterin (shScr) are aggregated. Are these organoids? I don‘t know! As described in the text, the culture vessels were coated with an agarose/agar-mix to prevent the attachment of the cells. Made by Chapper - unrestricted use allowed
Whether you can describe this as organoids could pro