An integrated brain-machine interface platform with thousands of channels
Elon Musk & Neuralink
Abstract
Brain-machine interfaces (BMIs) hold promise for the restoration of sensory and motor function and the treatment of neurological disorders, but clinical BMIs have not yet been widely adopted, in part because modest channel counts have limited their potential. In this white paper, we describe Neu-ralink’s first steps toward a scalable high-bandwidth BMI system. We have built arrays of small and flexible electrode “threads”, with as many as 3,072 electrodes per array distributed across 96 threads. We have also built a neurosurgical robot capable of inserting six threads (192 electrodes) per minute. Each thread can be individually inserted into the brain with micron precision for avoidance of sur-face vasculature and targeting specific brain regions. The electrode array is packaged into a small implantable device that contains custom chips for low-power on-board amplification and digitiza-tion: the package for 3,072 channels occupies less than( 23 × 18. 5 × 2 )mm^3. A single USB-C cable provides full-bandwidth data streaming from the device, recording from all channels simultaneously. This system has achieved a spiking yield of up to 85.5% in chronically implanted electrodes. Neu-ralink’s approach to BMI has unprecedented packaging density and scalability in a clinically relevant package.
1 Introduction
Brain-machine interfaces (BMIs) have the potential to help people with a wide range of clinical disorders. For example, researchers have demonstrated human neuroprosthetic control of computer cursors [1, 2, 3], robotic limbs [4, 5], and speech synthesizers [6] using no more than 256 electrodes. While these successes suggest that high fidelity information transfer between brains and machines is possible, development of BMI has been critically limited by the inability to record from large numbers of neurons. Noninvasive approaches can record the average of millions of neurons through the skull, but this signal is distorted and nonspecific [7, 8]. Invasive electrodes placed on the surface of the cortex can record useful signals, but they are limited in that they average the activity of thousands of neurons and cannot record signals deep in the brain [9]. Most BMI’s have used invasive techniques because the most precise readout of neural repre- sentations requires recording single action potentials from neurons in distributed, functionally-linked ensembles [10].
Microelectrodes are the gold-standard technology for recording action potentials, but there has not been a clinically- translatable microelectrode technology for large-scale recordings [11]. This would require a system with material prop- erties that provide high biocompatibility, safety, and longevity. Moreover, this device would also need a practical surgi- cal approach and high-density, low-power electronics to ultimately facilitate fully-implanted wireless operation.
Most devices for long-term neural recording are arrays of electrodes made from rigid metals or semiconductors [12, 13, 14, 15, 16, 17, 18]. While rigid metal arrays facilitate penetrating the brain, the size, Young’s modulus and bending stiff- ness mismatches between stiff probes and brain tissue can drive immune responses that limit the function and longevity of these devices [19, 11]. Furthermore, the fixed geometry of these arrays constrains the populations of neurons that can be accessed, especially due to the presence of vasculature.
An alternative approach is to use thin, flexible multi-electrode polymer probes [20, 21]. The smaller size and increased flexibility of these probes should offer greater biocompatibility. However, a drawback of this approach is that thin poly- mer probes are not stiff enough to directly insert into the brain; their insertion must be facilitated by stiffeners [22, 21], injection [23, 24] or other approaches [25], all of which are quite slow [26, 27]. To satisfy the functional requirements for a high-bandwidth BMI, while taking advantage of the properties of thin-film devices, we developed a robotic approach,
It is made available under a CC-BY-ND 4.0 International license.
was not peer-reviewed) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity.
July 16, 2019
where large numbers of fine and flexible polymer probes are efficiently and independently inserted across multiple brain regions [28].
Here, we report Neuralink’s progress towards a flexible, scalable BMI that increases channel count by an order of mag- nitude over prior work. Our system has three main components: ultra-fine polymer probes (section 2 of this report), a neurosurgical robot (section 3), and custom high-density electronics (section 4). We demonstrate the rapid implanta- tion of 96 polymer threads, each thread with 32 electrodes, in a( 4 × 7 )mm^2 area of brain for a total of 3,072 electrodes.
We developed miniaturized custom electronics that allow us to stream full broadband electrophysiology data simul- taneously from all these electrodes (section 5). We packaged this system for long-term implantation and developed custom online spike detection software that can detect action potentials with low latency. Together, this system serves as a state-of-the-art research platform and a first prototype towards a fully implantable human BMI.
2 Threads
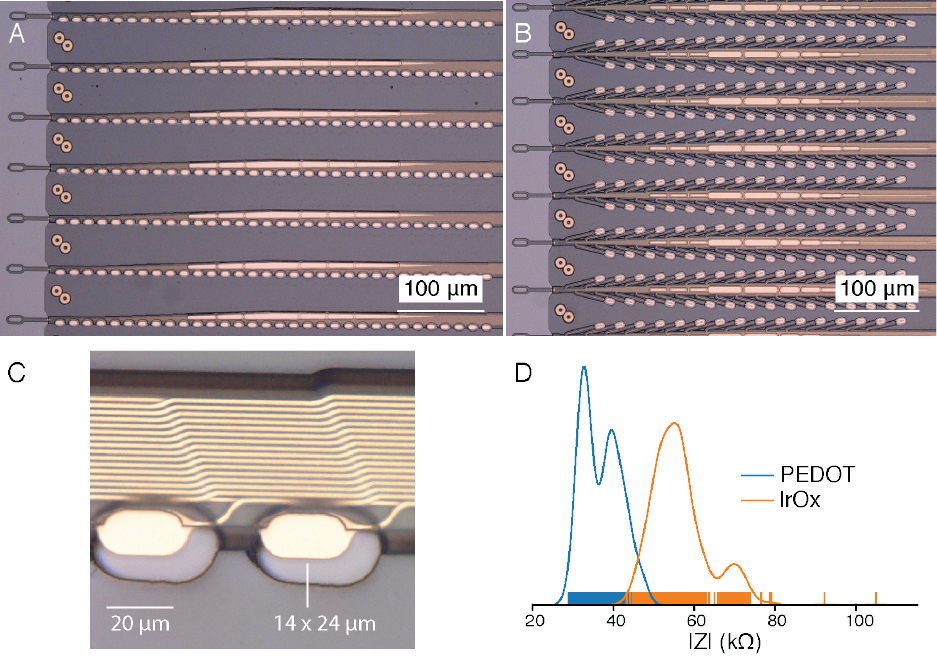
Figure 1: Our novel polymer probes. A. “Linear Edge” probes, with 32 electrode contacts spaced by 50μm. B. “Tree” probes with 32 electrode contacts spaced by 75μm. C. Increased magnification of individual electrodes for the thread design in panel A, emphasizing their small geometric surface area. D. Distribution of electrode impedances (measured at 1kHz) for two surface treatments: PEDOT (n=257) and IrOx (n=588).
We have developed a custom process to fabricate minimally displacive neural probes that employ a variety of biocom- patible thin film materials. The main substrate and dielectric used in these probes is polyimide, which encapsulates a gold thin film trace. Each thin film array is composed of a “thread” area that features electrode contacts and traces and a “sensor” area where the thin film interfaces with custom chips that enable signal amplification and acquisition. A wafer-level microfabrication process enables high-throughput manufacturing of these devices. Ten thin film devices are patterned on a wafer, each with 3,072 electrode contacts.
Each array has 48 or 96 threads, each of those containing 32 independent electrodes. Integrated chips are bonded to the contacts on the sensor area of the thin film using a flip-chip bonding process. One goal of this approach is to maintain
It is made available under a CC-BY-ND 4.0 International license.
was not peer-reviewed) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity.
July 16, 2019
a small thread cross-sectional area to minimize tissue displacement in the brain. To achieve this, while keeping channel count high, stepper lithography and other microfabrication techniques are used to form the metal film at sub-micron resolution.
We have designed and manufactured over 20 different thread and electrode types into our arrays; two example designs are shown in panels A and B of fig. 1. We have fabricated threads ranging from 5 to 50μm in width that incorporate recording sites of several geometries (fig. 1). Thread thickness is nominally 4 to 6μm, which includes up to three layers of insulation and two layers of conductor. Typical thread length is approximately 20mm. To manage these long, thin threads prior to insertion, parylene-c is deposited onto the threads to form a film on which the threads remain attached until the surgical robot pulls them off. Each thread ends in a( 16 × 50 )μm^2 loop to accommodate needle threading.
Since the individual gold electrode sites have small geometric surface areas (fig. 1C), we use surface modifications to lower the impedance for electrophysiology and increase the effective charge-carrying capacity of the interface (fig. 1D). Two such treatments that we have used are the electrically conductive polymer poly-ethylenedioxythiophene doped with polystyrene sulfonate (PEDOT:PSS) [29, 30] and iridium oxide (IrOx) [31, 32]. In bench-top testing we have achieved impedances of 36. 97 ± 4 .68kΩ (n=257electrodes) and 56. 46 ± 7 .10kΩ (n=588) for PEDOT:PSS and IrOx, respectively. The lower impedance of PEDOT:PSS is promising, however the long-term stability and biocompati- bility of PEDOT:PSS is less well established than for IrOx. These techniques and processes can be improved and further extended to other types of conductive electrode materials and coatings.
3 Robot
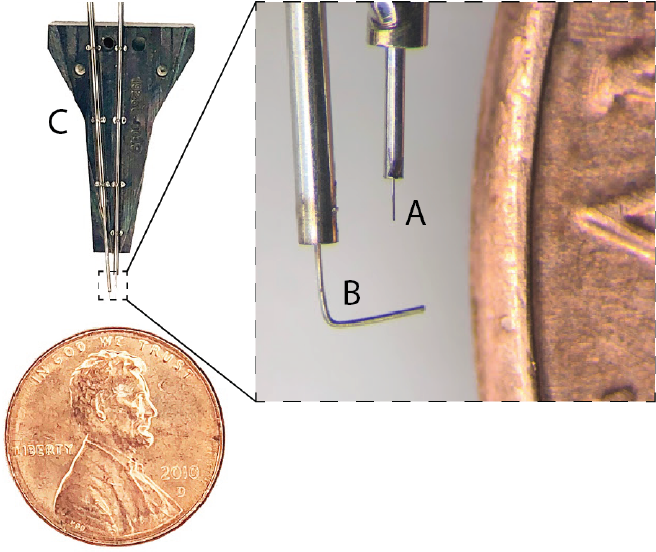
Figure 2: Needle pincher cartridge (NPC) compared with a penny for scale. A. Needle. B. Pincher. C. Cartridge.
Thin-film polymers have previously been used for electrode probes [21], but their low bending stiffness complicates insertions. Neuralink has developed a robotic insertion approach for inserting flexible probes [28], allowing rapid and reliable insertion of large numbers of polymer probes targeted to avoid vasculature and record from dispersed brain regions. The robot’s insertion head is mounted on 10μm globally accurate, 400mm×400mm×150mm travel three- axis stage, and holds a small, quick-swappable, “needle-pincher” assembly (fig. 2, fig. 3A).
The needle is milled from 40μm diameter tungsten-rhenium wire-stock electrochemically etched to 24μm diameter along the inserted length (fig. 2A). The tip of the needle is designed both to hook onto insertion loops—for transporting and inserting individual threads—and to penetrate the meninges and brain tissue. The needle is driven by a linear motor allowing variable insertion speeds and rapid retraction acceleration (up to 30,000mms−^2 ) to encourage separation of
It is made available under a CC-BY-ND 4.0 International license.
was not peer-reviewed) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity.
July 16, 2019
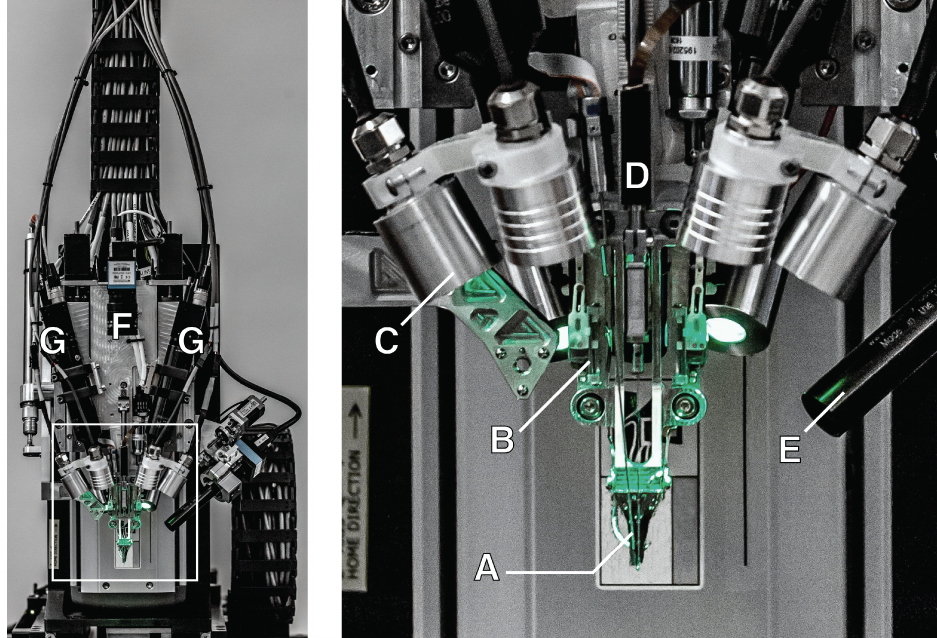
Figure 3: The robotic electrode inserter; enlarged view of the inserter-head shown in the inset. A. Loaded needle pinchercartridge. B. Low-forcecontactbrainpositionsensor. C. Lightmoduleswithmultipleindependentwavelengths. D. Needle motor. E. One of four cameras focused on the needle during insertion. F. Camera with wide angle view of surgical field. G. Stereoscopic cameras.
the probe from the needle. The pincher is a 50μm tungsten wire bent at the tip and driven both axially and rotationally (fig. 2B). It serves as support for probes during transport and as a guide to ensure that threads are inserted along the needle path. Figure 4 shows a sequence of photographs of the insertion process into an agarose brain proxy.
The inserter head also holds an imaging stack (fig. 3E–G) used for guiding the needle into the thread loop, insertion tar- geting, live insertion viewing, and insertion verification. In addition, the inserter head contains six independent light modules, each capable of independently illuminating with 405nm, 525nm and 650nm or white light (fig. 3C). The 405nm illumination excites fluorescence from polyimide and allows the optical stack and computer vision to reliably localize the( 16 × 50 )μm^2 thread loop and execute sub-micron visual servoing to guide, illuminated by 650nm the nee- dle through it. Stereoscopic cameras, software based monocular extended depth of field calculations, and illumination with 525nm light allow for precise estimation of the location of the cortical surface.
The robot registers insertion sites to a common coordinate frame with landmarks on the skull, which, when combined with depth tracking, enables precise targeting of anatomically defined brain structures. An integrated custom software suite allows pre-selection of all insertion sites, enabling planning of insertion paths optimized to minimize tangling and strain on the threads. The planning feature highlights the ability to avoid vasculature during insertions, one of the key advantages of inserting electrodes individually. This is particularly important, since damage to the blood-brain barrier is thought to play a key role in the brain’s inflammatory response to foreign objects [33].
The robot features an auto-insertion mode, which can insert up to 6 threads (192 electrodes) per minute. While the entire insertion procedure can be automated, the surgeon retains full control, and if desired, can make manual micro- adjustments to the thread position before each insertion into the cortex. The neurosurgical robot is compatible with sterile shrouding, and has features to facilitate successful and rapid insertions such as automatic sterile ultrasonic clean- ing of the needle. The needle pincher cartridge (NPC; fig. 2C) is the portion of the inserter head that makes direct contact with brain tissue and is a consumable that can be replaced mid-surgery in under a minute.
It is made available under a CC-BY-ND 4.0 International license.
was not peer-reviewed) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity.
July 16, 2019
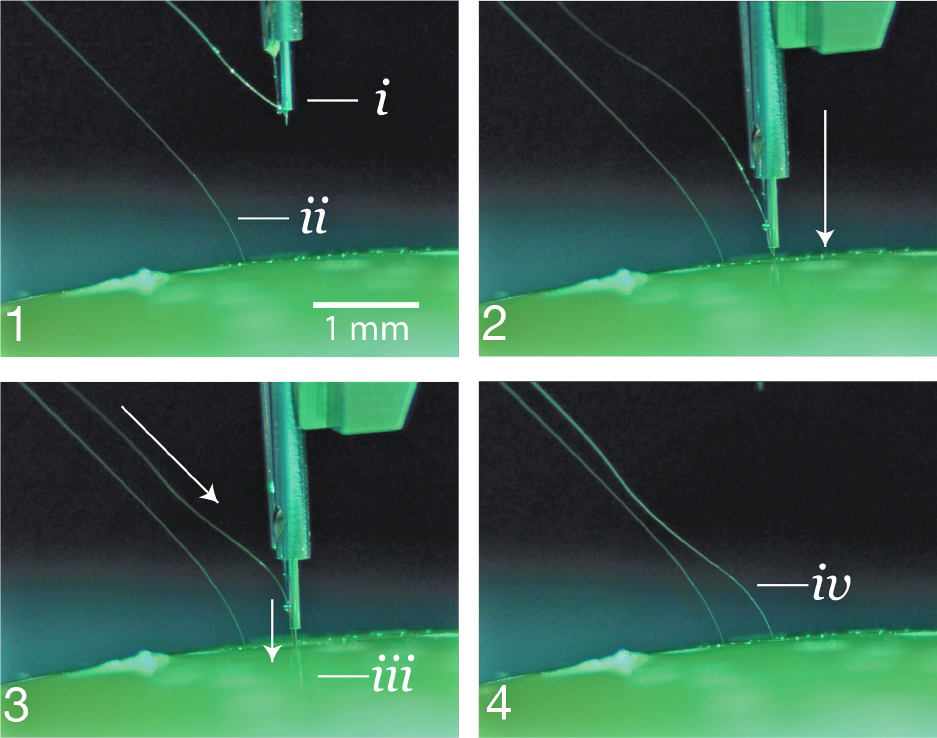
Figure 4: 1. The inserter approaches the brain proxy with a thread.i.needle and cannula.ii.previously inserted thread.
2. Inserter touches down on the brain proxy surface. 3. Needle penetrates tissue proxy, advancing the thread to the desired depth.iii.inserting thread. 4. Inserter pulls away, leaving the thread behind in the tissue proxy.iv.inserted thread.
With this system, we have demonstrated an average of 87. 1 ± 12 .6% (mean±s.d.) insertion success rate over 19 surg- eries. In this study, precise manual adjustments were made to avoid microvasculature on the cortical surface, slowing total insertion time from the fastest possible. Even with these adjustments, the total insertion time for this study av- eraged∼45min, for an approximate insertion rate of 29.6 electrodes per minute (fig. 6). Insertions were made in a ( 4 × 7 )mm^2 bilateral craniotomy with>300μm spacing between threads to maximize cortical coverage. This demon- strates that robotic insertion of thin polymer electrodes is an efficient and scalable approach for recording from large numbers of neurons in anatomically defined brain regions.
4 Electronics
Chronic recording from thousands of electrode sites presents significant electronics and packaging challenges. The den-
sity of recording channels necessitates placing the signal amplification and digitization stack within the array assembly,
otherwise the cable and connector requirements would be prohibitive. This recording stack must amplify small neural
signals (<10μVRMS) while rejecting out-of-band noise, sample and digitize the amplified signals, and stream out the
results for real-time processing—all using minimal power and size.
The electronics are built around our custom Neuralink application specific integrated circuit (ASIC), which consists of
256 individually programmable amplifiers (“analog pixels”), on-chip analog-to-digital converters (ADCs), and periph-
eral control circuitry for serializing the digitized outputs. The analog pixel is highly configurable: the gains and filter
It is made available under a CC-BY-ND 4.0 International license.
was not peer-reviewed) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity.
July 16, 2019
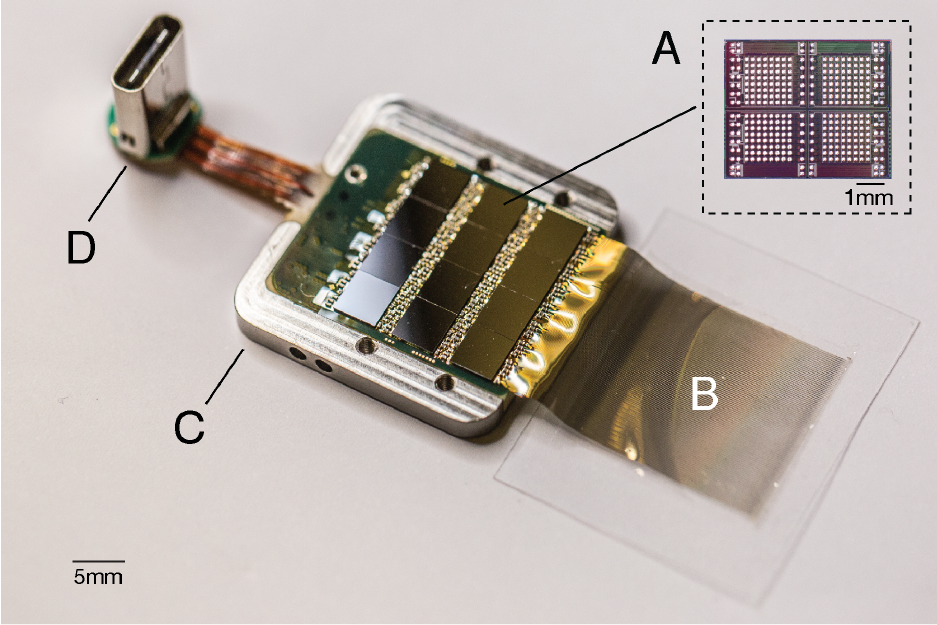
Figure 5: A packaged sensor device. A. individual neural processing ASIC capable of processing 256 channels of data. Thisparticularpackageddevicecontains12ofthesechipsforatotalof3,072channels. B. Polymerthreadsonparylene-c substrate. C. Titanium enclosure (lid removed). D. Digital USB-C connector for power and data.
properties can be calibrated to account for variability in signal-quality due to process variations and the electrophysio- logical environment. The on-chip ADC samples at 19.3kHz with 10bit resolution. Each analog pixel consumes 5.2μW and the whole ASIC consumes∼6mW, including the clock drivers. Performance of the Neuralink ASIC is summarized in table 1 and a photograph of the fabricated device is shown in fig. 5A.
The Neuralink ASIC forms the core of a modular recording platform that allows for easy replacement of constitutive parts for research and development purposes (fig. 5). In the systems discussed here, a number of ASICs are integrated into a standard printed circuit board (PCB) using flip-chip integration. Each system consists of a field-programmable gate array (FPGA); real-time temperature, accelerometer, and magnetometer sensors; and a single USB-C connector for full-bandwidth data transfer. The systems are packaged in titanium cases which are coated with parylene-c, which serves as a moisture barrier to prevent fluid ingress and prolong functional lifetime.
We describe two such configurations that we have built, a 1,536 channel recording system (“System A”) and a 3, 072 channel recording system (“System B”), summarized in table 2. While System A employs the current-generation Neu- ralink ASIC, System B uses an earlier revision with comparable functionality but poorer performance specifications. System B was designed to maximize channel density and is used for applications that demand extremely high channel count. In contrast, System A was designed to facilitate faster and more reliable manufacturing; it can be built five times faster than System B with better yields.
An ethernet-connected base station converts the data streams from these systems into multicast 10G ethernet UDP packets allowing downstream users to process the data in a variety of ways, e.g. visualizing the data in real-time or writing it to disk. Each base station can connect to up to three implants simultaneously. These devices are further supported by a software ecosystem that allows for plug and play usability with zero configuration: neural data begins streaming automatically when a cable is connected.
It is made available under a CC-BY-ND 4.0 International license.
was not peer-reviewed) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity.
July 16, 2019

Table 1: Neuralink ASIC
Number of Channels 256
Gain 42 .9–59.4dB
Bandwidth 3Hz–27kHz
Input-referred noise (3Hz - 10KHz) 5 .9μVRMS
Maximum Differential Input Range 7 .2mVPP
ADC resolution 10bit
Analog Pixel Power 5 .2μW
Table 2: Two recording system configurations
System A System B
Number of Channels 1 , 536 3 , 072
Sampling Rate 19 .3kHz 18 .6kHz
Total System Power Consumption 550mW 750mW
Total System Size ( 24. 5 × 20 × 1. 65 )mm^3 ( 23 × 18. 5 × 2 )mm^3
5 Electrophysiology
We have implanted both systems A and B in male Long-Evans rats, as described in section 3. All animal procedures were performed in accordance with the National Research Council’sGuide for the Care and Use of Laboratory Animalsand were approved by the Neuralink Institutional Animal Care and Use Committee. Electrophysiological recordings were made as the animals freely explored an arena equipped with a commutated cable that permi