The immune cells - the superheroes in our body fight the invaders. But of course to fight they need to eat. Like any other cell in the body, they get nutrients from the food we eat. Cells including immune cells respond to nutrients or their lack. But how do immune cells choose to respond to it? Do they age gracefully in a lower energy supply? In this episode, we will explore the effect of caloric restriction on immune cells and how it slows down ageing if it does.
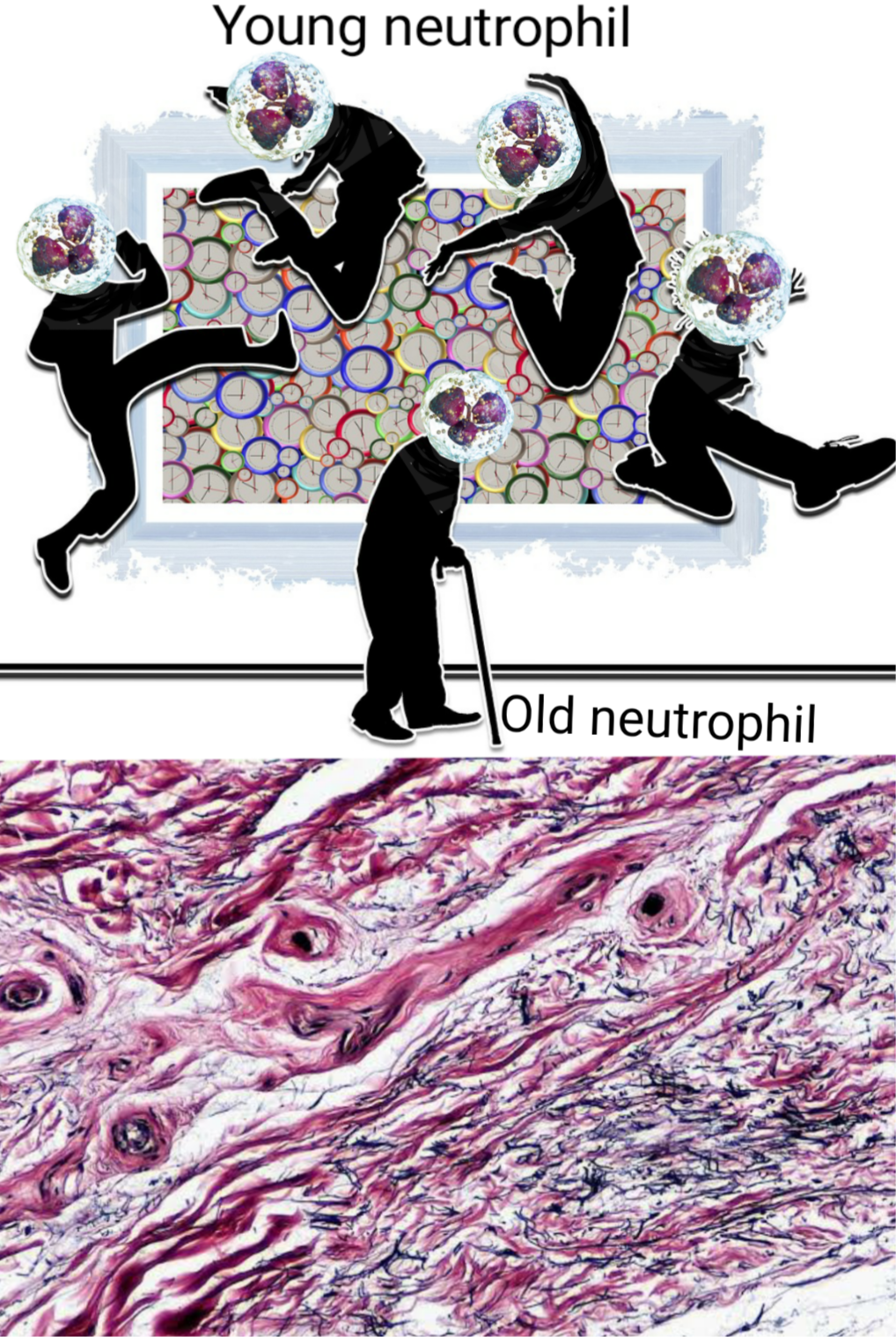
Old age impairs immune cells, just like it impairs us. The image shows how a young neutrophil when called up, enters a tissue, spends some time and then clears its way fuck out of the tissue. While an old neutrophil lingers its way through, have difficulty coming in and going out, among other things. Illustrated by @scienceblocks using following images - neutrophil by Blausen.com staff (2014). "Medical gallery of Blausen Medical 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436. | CC by 3.0 Age youth contrast by Geralt. | Pixabay Connective tissue by Berkshire Community College Bioscience Image Library | Public Domain
Recap
Till this point in the series we saw - how DNA damage and perturbed DNA repair pathways contribute to ageing (Also see this blog). We also saw how mitochondria, the cell organelle that derives energy out of food using oxygen, also contributes to the process of ageing. The mitochondria become less efficient as we age. They produce more ROS and contribute to cellular damage. Its biogenesis and fission and fusion dynamics also get perturbed. It also affects cell renewal via stem cells. We also saw that caloric restriction can slow down ageing. We saw that cutting off a few calories from the diet causes the cells to sense the lack of nutrition. Caloric restriction activates/inactivate different pathways - including but not limited to mTOR, AMPK, and sirtuins which then affects the stuff mentioned above - it increases DNA repair, decreases damage caused by mitochondria, and even improves mitochondrial morphogenesis. In our quest, we also explored certain molecules that can mimic the effect of caloric restriction. However, we knew that we still don't understand the complete story of ageing. Hence, we dug further.
In the most recent episode, we shifted our focus from damage that arises from within the cells to that which arise from outside. We talked about the chronic low-grade inflammation that increases with ageing. We also tried to dwell on possible reasons behind it. We talked about the damaged molecules and senescent cells that increase with age and tend to recruit more inflammation-causing immune cells. We also realized that the immune system itself ages. We saw that the immune cells differentiation from hematopoietic stem cells is affected. The way they migrate, the receptors express and things they secrete are all prone to the course of ageing. However, what we did not do is look at how caloric restriction affects this ageing immune system and inflammation if it does. In this episode, this is what we are going to explore and figure out some more elixirs of life.
A Tale of Inflammation and Caloric Restriction.
What we know is that many age-associated diseases also have an inflammation component. Hence, to know whether the caloric restriction has any effect on improving inflammation status, we should ask what happens to these diseases on a calorie-restricted diet. Caloric restriction has been shown to have protective effect or delay the onset of the chronic inflammatory diseases - such as type 2 diabetes, hypertension, osteoarthritis, cardiovascular diseases, and even cancer (Radakovich et al., 2019, Omodei et al., 2011).
Also, since autoimmune diseases are classic examples of inflammation gone haywire, and their incidence increases with age - it would be interesting to ask if caloric restriction does play a role in soothing them or delaying them. In 1992, Kubo et al.,, fed NZB mice (a mice model for autoimmune disease development) with a calorie-restricted diet. They observed that it prevented the onset of autoimmunity in these mice - it normalized the T and B cell number. It normalized the size of the spleen and also had an effect on non-B and non-T lymphoid cell numbers. Then in 2016, Choi et al.,, tested a fast mimicking diet in mice model for multiple sclerosis. They found that this diet was helpful not only in ameliorating the symptoms of the disease but also in reversing some of the damage. Moreover, they also did a small trial on human patients and got some exciting preliminary results.
Though, it was observed in these studies that at least a part of this effect is mediated via apoptosis of autoimmune T and B cells. However, what is not clear is that if it was a direct effect on ageing phenotype of the immune system or indirect effect on some other cells. In order to know this, we need to see if the caloric restriction has any effect on infammgeing sources that we discussed earlier. Let's see, shall we?
Does calorie restriction counter Immunosenescence?
What happens to hungry T cells?
In the last blog, we saw that the immune system gets old as well - aka immunosenescence. The innate immune cells overpower adaptive immune cells. Well, because there is a decrease in the number of naive T cells and B cells. However old senescent T cells which have short telomeres and reduced proliferation capacity accumulate. The environment of thymus, where T cells differentiate also changes (Duggal, 2018) - Thymus is where T cells mature, where its decided if they will be helper T cells or cytotoxic T cells, where they learn the difference between self and foreign, where they trained to be tolerant or offensive against certain molecular patterns on other cells and pathogens. Now just imagine the dread of the immune system if this sacred organ changes with time. As if getting new lymphocytes(T and B cells) from bone marrow was difficult enough in the old body, now we also have issues in an organ where they mature.
Nevertheless, it has been observed that thymic adipogenesis (one of the phenotypes associated with ageing thymus) is reduced by caloric restriction. The caloric restricted mice show more intact and functional thymus, high expression of IL2, and high proliferation of T cells as compared to their age-matched controls (Yang et al 2009). Furthermore, the caloric restriction also seems to take care of old T cells. Spaulding et al., 1997 showed that the old mice are worse at getting rid of damaged T cells via irradiation, heat shock or chemical damage. However, the caloric restriction made them efficient at it. And not only caloric restriction made them efficient at removing damaged T cells but it also inhibited them from becoming senescent, to begin with (Messaoudi et al., 2006). Plus that increased IL2 in the thymus, that helps regulate metabolic profile of T cells and promote T regulatory cell development (T reg cells helps put a break on other T cells when their job is done). Moreover, in mice on a calorie-restricted diet it appears that last stage advanced differentiation of T cells and even natural killer (NK) cells are inhibited (White et al., 2017). This kind of keeps the pool of naive cells ready, while at the same time inhibiting cynical old guys to survive too long and cause damage (which may even prevent any age-associated self-destruction).
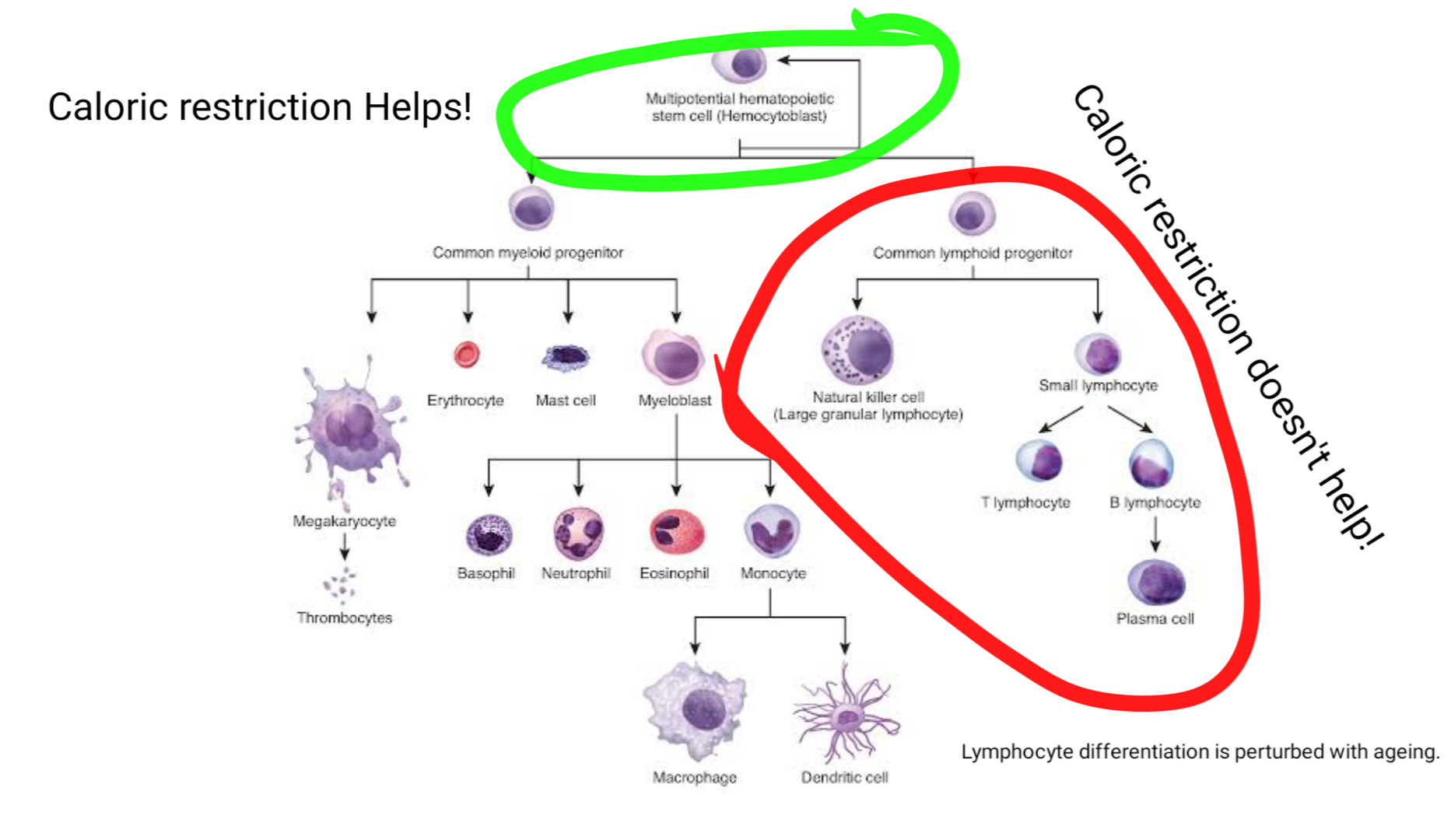
Image showing all the cells made in bone marrow by the hematopoietic stem cells. The lymphocyte differentiation arm is affected by ageing. This defect in this arm is not improved via caloric restriction. However, calorie restriction does preserve the hematopoietic stem cells Adapted from Hematopoiesis by OpenStax | CC BY 4.0
However, despite all the good effects of caloric restriction it does not seem to have any benefit on rescuing lymphoid differentiation of HSCs, that we saw in the last blog is also impaired with ageing. It kind of slows down the ageing of hematopoietic stem cells by keeping them in a quiescent state. At first look, this may seem good, but if you think about it then if HSCs don't respond adequately to pathogen and other stressors this might be dangerous outside sterile conditions of the lab (Tang et al., 2016).
This may indicate three things - one being that caloric restriction is not sufficient to reverse all the effects of ageing and might need supplementation via other nutrients or drug to make it more optimal. Second, that rescue of autoimmune disease symptoms might be limited to caloric restriction killing autoimmune lymphoid cells and third that CR might also mellow down cells of the innate immune system which causes some of the damage.
The innate immunity on diet.
Can we neuter the bonkers neutrophils?
Talking of innate immune cells, remember I told you about neutrophils in the last blog. The immune cells that are first to arrive at the site of injury or infection. However, when they overstay their welcome they tend to cause tissue damage aggravating the inflammation further. We also learnt that during ageing the neutrophils tend to have defective migration both into and out of the tissue.
Their adhesion receptor profile also changes. It appears that Beta 2 integrin in aged neutrophils binds more strongly to ICAM-1. This may make them adhere strongly to the tissue they invade into and may also prolong their survival. It comes as no surprise their death via apoptosis is not like normal young cells making them overstay their welcome. Moreover, neutrophils are produced in bone marrow, and then after some rounds in circulation, they return to bone marrow and die. However, that going to bone marrow and dying part doesn't work out in aged neutrophils. Well, if they don't go and die they have to go somewhere. Maybe, that's one of the reasons why there is unwanted low-grade inflammation in aged tissues. (Qian et al., 2014, Kolaczkowska, 2016). We also saw that their profile changes to a more damaging kind.
Fasting, on the other hand, has been shown to be beneficial in reducing inflammation in diseases such as rheumatoid arthritis. It is likely that at least part of this effect is mediated by targeting the neutrophil function - such as secretion of inflammatory cytokines. For instance, Hafström, in 1988, showed that fasting reduces leukotriene B4 production in neutrophils - a compound known to promote neutrophil migration and aggregation. If you think about it, one of the early pathway discovered to be regulated by caloric restriction is insulin-like growth factor/PI3K pathway. The caloric restriction seems to inhibit the PI3K/AKT pathway (Mercken et al., 2013). In older adults neutrophil shows high consecutive activation of PI3K in neutrophils (Naccache et al., 2014). Inhibiting PI3K using a pharmacological inhibitor for 2 isoforms of PI3K improves neutrophil migration accuracy in old neutrophils (Sapey et al., 2014). On the other hand, the aged neutrophils seem to employ p38 MAPK and TLR4 pathway to create their altered adhesion molecule and cytokine profile. And inhibition of these pathways seem to improve neutrophil function (Uhl et al., 2016).
In fact, recently Wu et al., showed that dietary restriction mediates an effect on innate immunity in C elegans via the p38 pathway. Too much of p38 activation caused hyperresponsive innate immune system, and dietary restriction got things to normal. However, when p38 was completely inhibited the lifespan was not extended despite caloric restriction. Indicating that the pathway needs to work at some optimal level for the innate immune system to behave. It would be interesting to a drug targeting p38 or PI3K specifically in immune cells can serve as the elixir of life or not?
How to get senile Macrophages back on track?
However, neutrophils are not the only cell of the innate immune system who lose their shit in old age. Macrophage, the next important innate immune cell come at the site of injury or infection also show increased cytokine production, esp TNFa and IL6. If you are wondering then TNFa is the same molecule against which biologicals are designed in treatment of autoimmune diseases. They too have defective chemotaxis and reduced phagocytotic activity. In a nutshell, they become this cynical person who hurt you by words but does little to tackle real issues. However, the caloric restriction comes to rescue once again.
For instance, in 2018, Trott et al., showed that macrophage infiltration into walls of the artery, along with senescent B and T cells increase with ageing. Given the macrophages forms the foam cells along with all the fat they eat in walls of arteries to cause arteriosclerosis; their accumulation may explain why t